Biohaven: Insider Trading, Financial Developments, and Clinical Advances

Biohaven (BHVN:US), a forward-thinking biopharmaceutical company, is dedicated to developing treatments for neurological and neuropsychiatric diseases. Listed on the New York Stock Exchange, Biohaven has a market capitalization of $3.054 billion.
Insider Buying
Recently, insider buying activity at the company has been notable. Between May 13 and 30, 2024, board members John W. Childs and Gregory Hugh Bailey purchased 71,500 shares of Biohaven at an average price of $34.98 per share, totaling an investment of $2.50 million. Additionally, in late April 2024, Bailey made a significant investment of around $1 million.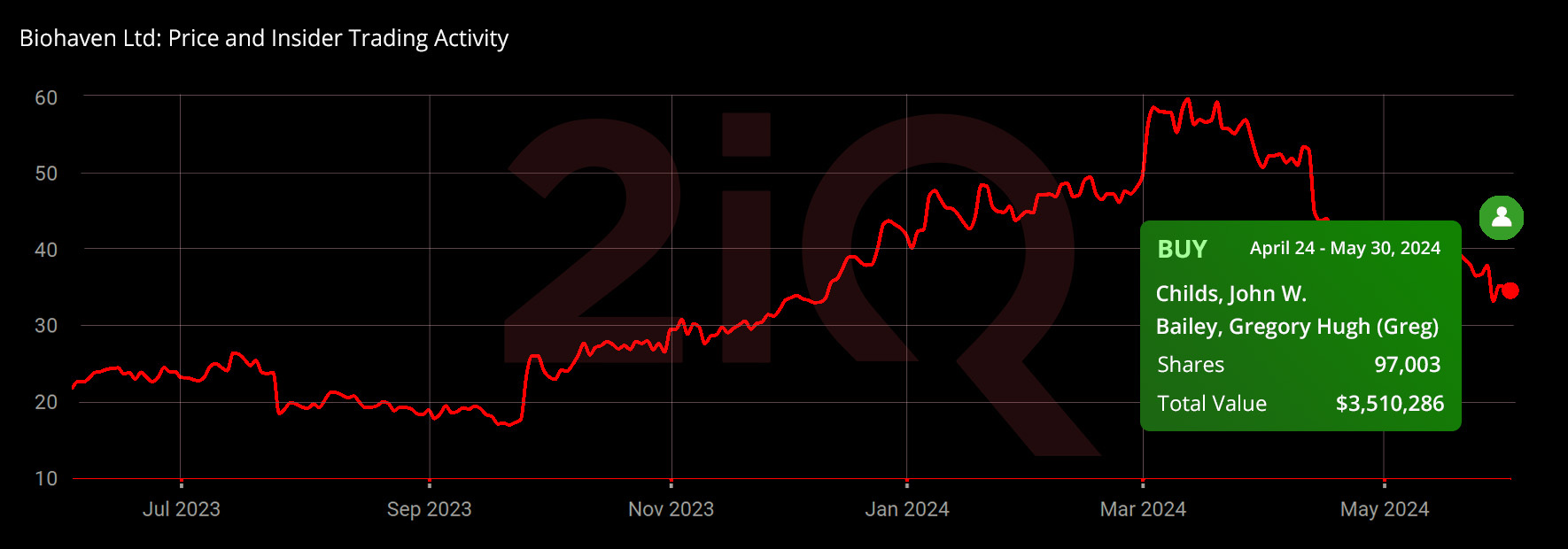
Clinical Advancements
Biohaven is making strides in its clinical trials, particularly with the Phase I/II study of BHV-1510, a Trop-2-directed antibody-drug conjugate (ADC) for advanced epithelial cell tumors. This first-in-human, multi-center, open-label trial aims to determine the safety and recommended doses for expansion and evaluate the drug's preliminary efficacy.
Financial Performance and Development
Biohaven reported a strong financial position in its first-quarter 2024 results. As of March 31, 2024, the company held cash, equivalents, marketable securities, and restricted cash totaling approximately $287.6 million. This excludes the net proceeds of approximately $247.8 million from a public offering completed on April 22, 2024. This offering saw the sale of 6,451,220 common shares at $41.00 per share.
Recent business highlights include the completion of Phase 2 and 3 programs for epilepsy, major depressive disorder (MDD), and bipolar disorder with BHV-7000. The FDA granted rare pediatric disease designation for taldefgrobep alfa, which could receive a priority review voucher if approved for spinal muscular atrophy (SMA). Additionally, Biohaven showcased significant developments in its Kv7 ion channel modulation and TRPM3 antagonism programs at the American Academy of Neurology (AAN) Annual Meeting.
Taking into account the company’s exceptional performance in neuroscience and immunology, and the trust that the insiders have shown in the firm, it won’t be wrong to say that the company is in synch for a potentially bullish future.
Latest Stories

GameStop Corp: Bold Moves, Insider Confidence and a Strong Future

Petco CEO Makes Major Insider Stock Purchases – A Bullish Signal?

74Software Board Member Makes Major Insider Purchase – A Bullish Signal?

Insider Cluster Buying at Energean PLC Signals Confidence Amid Strategic Developments

Redwire Corporation: Insiders Buying Amid a Stock Slump